Abstract
Background: Chronic red blood cell (RBC) transfusion is an established therapy to prevent stroke in patients with sickle cell anemia (SCA). Chronic transfusion, however, requires considerable blood resources and has risks so a strategy to decrease transfusion requirements is highly desirable especially given blood shortages. Chronic transfusion also can fail to prevent progressive cerebrovascular disease so further optimization of this therapy is needed. Hydroxyurea has been studied as an alternative to chronic transfusion, but alone it provides inadequate cerebrovascular protection for patients at highest risk for stroke. Combination therapy hydroxyurea and transfusion (HAT) may provide benefits over either therapy alone because each ameliorates the pathology of SCA via different mechanisms. To evaluate the feasibility and impact of HAT on transfusion requirements, we conducted a single-arm clinical trial (NCT03644953).
Methods: Pediatric patients with SCA already receiving simple chronic transfusion for stroke prevention for at least one year were approached. Patients with poor adherence to chronic transfusion defined by having a hemoglobin (Hb) S >45% and a transfusion interval >5 weeks in the last year were excluded. Enrolled patients were started on hydroxyurea 20 mg/kg/day and continued on simple chronic transfusions with the same transfusion protocol. Transfusion volumes were based on the pre-transfusion Hb to achieve a target post-transfusion Hb of 11.5-12.0 g/dL. Hydroxyurea was dose escalated to achieve a HAT target dose (HAT-TD) defined by either an absolute neutrophil count <4000/µL, or a pre-transfusion Hb >10g/dL with HbS >30%. Transfusion volumes and pre-transfusion laboratory values were compared the year before HAT and the year after HAT-TD.
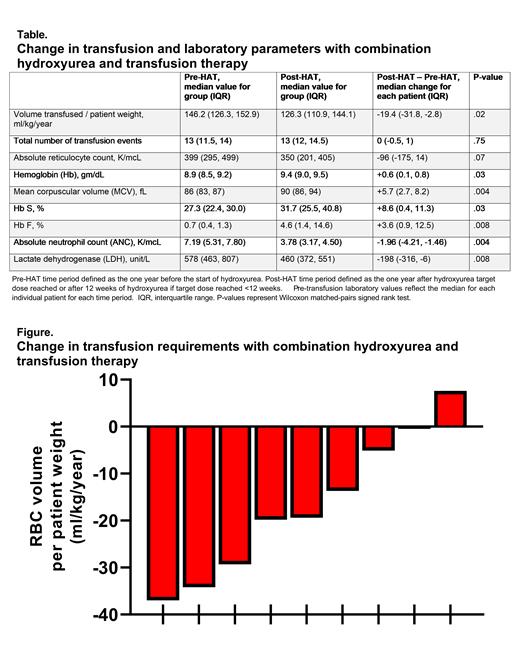
Results: A total of 19 eligible patients were offered participation and 14 enrolled (74% recruitment ratio). The median age was 11.2 years (range 5.3-19.8). The primary indication for chronic transfusion was history of an abnormal transcranial Doppler ultrasound (n=7), overt stroke (n=6), and progression of cerebral vasculopathy (n=1). Five participants ended the study prematurely (care transferred to another hospital, nonadherence to transfusions, patient desire to not take medications, hair thinning, family concern after a hospitalization); none had a serious adverse event that was attributed to HAT. Among the 9 participants who completed the study, the median HAT-TD was 25 mg/kg/dose (range 20-30) and was achieved after a median of 18 weeks (range 0-26). The median hydroxyurea adherence ratio, measured by pill counts, was 91%. One patient with baseline splenomegaly developed splenic sequestration requiring hospitalization; there were no other serious adverse events attributed to HAT. One patient developed a pre-transfusion Hb >11 g/dL and HbS >45%. This patient had hydroxyurea held for 12 weeks and was changed to partial manual exchange transfusions. With HAT, patients had a significant increase in pre-transfusion total Hb, HbS, HbF, and MCV; and a significant decrease in ANC and LDH (Table). After HAT-TD, 8/9 participants received fewer RBCs with a significant median RBC volume reduction of 19.4 ml/kg/year (p=.02, Figure). The single patient who received more RBCs after HAT-TD had suspected nonadherence to hydroxyurea returning only 33% of dispensed hydroxyurea bottles. While receiving HAT 8/9 patients had brain magnetic resonance imaging for clinical monitoring after a median of 75 weeks (range 29-100), no patient had a new infarct or progressive vasculopathy.
Conclusion: Hydroxyurea added to chronic transfusion therapy for patients with SCA is feasible. HAT effectively decreases transfusion volumes so can help conserve blood resources. While Hb S% increases with HAT, this increase is relatively small and markers of hemolysis improved. Most patients are able to safely continue simple transfusions when hydroxyurea is added, but a few may require exchange transfusions. The effect of HAT on cerebrovascular disease warrants further study.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal